A04: Life-death death decisions in response to mitotic errors
Project Leader: Andreas Villunger
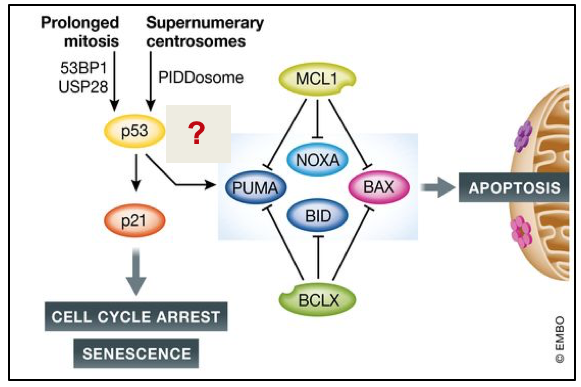
Decisions to undergo apoptosis are often linked to cell division. Errors in spindle pole formation, microtubule to kinetochore attachements or chromosome alignment cause delays in mitosis that promote erronenous sister chromatid separation or culminate in failed cell division (cytokinesis). Cytokinesis failure goes hand in hand with unscheduled whole genome duplication (WGD) and centrosome amplification. Extra centrosomes can create multipolar spindles that are again error prone. All these phenomena are known to increase the risk for chromosomal instability (CIN) and aneuploidy, both frequent phenomena in cancer. To avoid these tumor-promoting events, a cell fate decision-making process is initiated in response to delays in mitosis, WGD and centrosome accumulation. This ultimately causes cell cycle arrest and senescence or cell death. While the former clearly involves the p53 tumor suppressor, the latter fate does not depend on it and cell type-specific differences in decision-making have been observed. Genes defining cell type-dependent decision-making are still poorly characterized. We have shown that the PIDDosome, an activation platform for caspase 2, limits cell growth and cell survival in response to extra centrosomes, while the BCL-2 network defines cell fate in response to delays in mitosis. Characterizing new genes involved in decision-making in response to mitotic errors is at the heart of this proposal. First, we will study a set of genes involved in promoting cell death in response to mitotic errors that we have already identified in a CRISPR/Cas9 screen. p53-induced transcriptome changes linked to different cell fates will be defined by time-resolved RNA-sequencing experiments in the second part. In the third part, temporal changes in the transcriptome will be linked to p53 gene occupancy. Next to providing a valuable resource to the scientific community, we believe that this integrative approach will clarify why, when and how cells choose to arrest and survive or die in response to mitotic errors.
Funded by the Austrian Science Fund (FWF)
Publications
- Braun VZ, Karbon G, Schuler F, Schapfl MA, Weiss JG, Petermann PY, Spierings DCJ, Tijhuis AE, Foijer F, Labi V, Villunger A. Extra centrosomes delay DNA damage-driven tumorigenesis. Sci Adv. 2024 Mar 29;10(13):eadk0564. doi: 10.1126/sciadv.adk0564. Epub 2024 Mar 29. PMID: 38552015; PMCID: PMC10980279.

